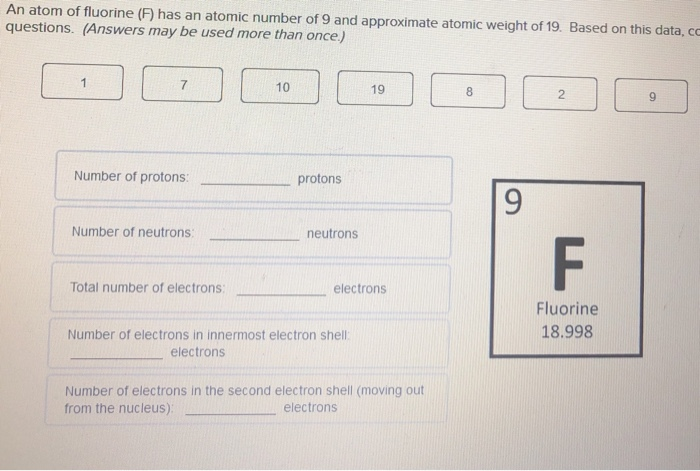
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Fluorine is 9. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
- The maximum electrons that can be carried by the sub-shell S is 2, by P is 6, by D is 10, and the F sub-shell can carry 14. This decides the electron capacity of the shells. The K shell contains a 1s subshell hence it can carry 2 electrons, the L shell has 2s and 2p, and can carry 8 electrons.
- Name: Fluorine Symbol: F Atomic Number: 9 Atomic Mass: 18.998404 amu Melting Point:-219.62 °C (53.530006 K, -363.31598 °F) Boiling Point:-188.14 °C (85.01 K, -306.652 °F) Number of Protons/Electrons: 9 Number of Neutrons: 10 Classification: Halogen Crystal Structure: Cubic Density @ 293 K: 1.696 g/cm 3 Color: Greenish Atomic Structure.
Electron Configurations Worksheet- Examples
- Read my article in Science Education based on my dissertation.



Electron Configuration Notation:
-shows the arrangment of electrons around the nucleus of an atom.
- helps chemist understanding how elements form chemical bonds.
- can be written using the period table or an electron configuration chart.
Skylum hdr.
Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. Blender shade smooth angle. The remaining five electrons will go in the 2p orbital. Therefore the F electron configuration will be 1s22s22p5.
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.

